Creighton’s Silver Bullet
The use of Creighton Dental’s silver fluoride stannous fluoride combination (CSDS TM) to control and diagnose caries is well recognised. Teeth treated with CSDSTM can form a hard black matte on the treated surface known, as “The Black Diamond”. Creighton Dental has supercharged “The Back Diamond”, to the form “The Surface Silver Bullet’’. The application of silver fluoride followed by stannous fluoride currently produces a black matte, a visual signal caries is not progressing. Years of research and development has enhanced the silver fluoride, and stannous fluoride components in a novel and inventive way to produce a “Supercharged Black Diamond”. Ostensibly both black diamonds appear to be identical, but what lies on the surface of the “Supercharged Black Diamond “ of a treated carious surface lesion is a biochemical phenomenon, a world first method of synthesising nanao silver particles (AgNPs) on a carious lesion in situ , orally under physiological conditions of temperature, and pressure resulting in “The Black Diamond” transforming into, “The Surface Silver Bullet” .
The Silver Bullet helps mitigate the problem that sometimes occurs i.e., the depletion of silver ions one of the active bactericidal forces in CSDS. The diminished power of a CSDS treatment can be heralded by a visual colour change , from black matte to a lightening or reversion to the original caries lesion colour of yellow/brown, as described by Dr Graham Craig . (Craig et al., 2013) The chemical reduction of a silver fluoride dental solution, the preferred metal precursor, with an enhanced stannous fluoride, the preferred metal salt, or an organic reductant, reduces silver ions (Ag+) to silver atoms (Ag 0), that then produce nano silver particles (AgNPs). These can serve as a reservoir for silver ions, revitalising the deactivation process of the microorganisms present on carious lesion surfaces. The antibacterial properties of AgNPs are well recognised in the dental field. The tooth surface treatment method described, fulfils the need to maintain the uncompromised clinical efficacy of silver fluoride dental solutions, and shows the important role of the stannous ions in Creighton Dental’s CSDS’s stannous fluoride component.
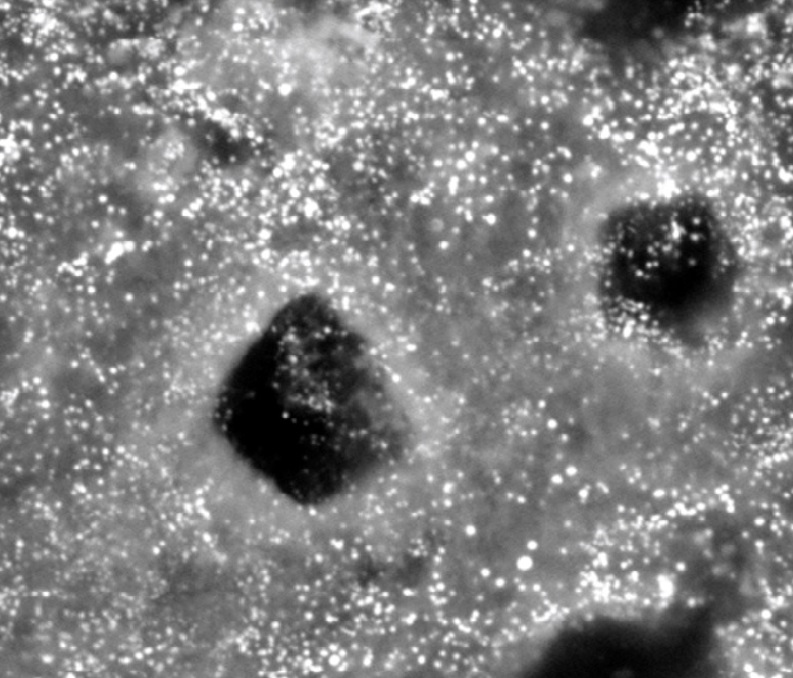
Fig 1 Scanning Electron Microscope (with a capability of nano size resolution) photograph of the “ Supercharged Black Diamond’ precipitate on a CSDS treated carious lesion, is inundated with white dots . The size of the white dots, and the fact they are silver, are consistent with nano silver .
Scanning Electron Microscope (SEM) sample images in Fig 1, are consistent with the white shining dots on the surface being nano silver particles (AgNPs).
Stannous fluoride has long been established as a topical fluoride with antimicrobial properties. The therapeutic efficacy of stannous fluoride depends on its stability. When free divalent (2+) stannous ions (Sn2+) in solution are oxidised to form tetravalent (4+) stannic ions (Sn4+), they no longer have protective properties. Stannous ions disassociate in water to form a white precipitate of tin (stannous) hydroxide, a process known as hydrolysis. All these chemical changes affect the bioavailability of stannous ions resulting in deactivation of an essential therapeutic ingredient.
Preparation of improved stannous fluoride (SnF2).
Creighton mitigates the inherent instability of the stannous ion, preparing stannous fluoride by dissolving 10%(w/v) stannous fluoride powder in between 1-100% (w/v) glycerol (glycerine), and between 1%-20% (w/v) sorbitol at a temperature of between 25-185 °C (degrees Celsius). This method produces a clear improved stabilised stannous fluoride dental solution with no white precipitate formation. This method produced an improved stable SnF2, which after age testing showed no observed change in stannous ion levels after aging at 55°C for 80 days, (the equivalent of 24 months of real time age testing in challenging humid and temperature environments), the stannous fluoride dental reductant solution was clear with no evidence of the characteristic white precipitate formed by oxidation of stannous ions.